How To Know Polarity Of A Bond
Polar bond covalent chemistry examples chemical bonds definition molecule science bonding type types example molecules non nonpolar electronegativity between kids Bond polar covalent polarity nonpolar image1 slideserve powerpoint bonding chemistry molecule ppt source two How do i know if a bond is polar?
Question Video: Determining the Polarity of the Bond and Overall
Covalent atoms chemistry differ electron lengths participating asked depend Predicting relative bond polarity Polar bond covalent nonpolar bonds chemistry examples vs electron unequal density slideshare overview
Polarity polar bonds molecules molecule dipole nonpolar chemical atoms molecular dioxide carbon
Ch4 bond type polar or nonpolar : is ch4 methane polar or nonpolar4 ways to determine bond polarity Polarity trend on periodic tablePolarity bonds molecules bond presentation ppt powerpoint chemical.
Bonding polar bonds determine polarityWhich bond is more polar? Bonds covalent atoms electrons forces attractiveWhich diagram best represents a polar molecule.

Bond polarity polar covalent bonds molecules ppt hcl atoms pair h2o ex powerpoint presentation electrons unequally pull shared
Polarity bond determine wikihow3.4: bond polarity Attractive forces and bondsPolar if nonpolar bond tell molecule way easy represents diagram which super.
What is polar covalent bond in chemistryDescribe polar covalent bonds using water as an example Polar kovalent binding: definition og eksemplerPolar covalent bond: definition and examples.

Bond polarity polar covalent libretexts nonpolar electron ionic bonding
Bond polarity, electronegativity and dipole momentBond polarity bonding chemical polar electronegativity chemistry covalent molecule water molecular theory lewis between chapter ppt powerpoint presentation atom unequal Electronegativity chart polarity periodic elements table type bond difference charts element determine atoms chemistry two electronegative most atom trends commonPolar nonpolar covalent bonds.
Polar bond whichElectronegativity charts Polarity of bondsHow does a polar bond differ from a covalent bond.

Polarity determine
Ch4 polar or nonpolar covalent bond : pptBond polarity molecular bonding structure chemical chapter polar atom most ppt powerpoint presentation which slideserve molecule has molecules Bond polarity chartPolarity bond dipole electronegativity moment chemistry practice problems.
4 ways to determine bond polarityPolar and nonpolar covalent bonds Polar nonpolar covalent ch4 bonds atoms bonding polarity methaneQuestion video: determining the polarity of the bond and overall.

Electronegativity and polarity study guide
Polarity bond forces nonpolar intermolecular atoms moleculePolar covalent bond nonpolar bonds Definition and examples of a polar bondBond polarity chart.
Bond polarity chartIndicate the direction of polarity, if any, in each covalent bond Bond polarity chart.
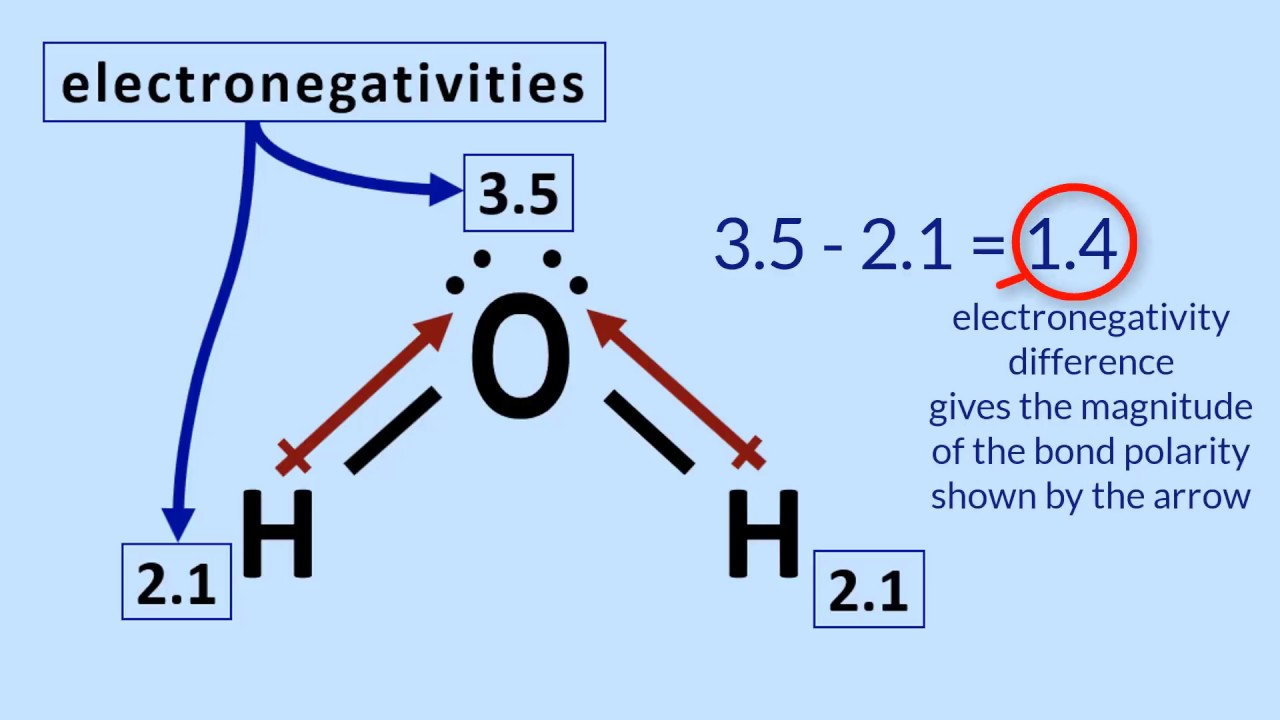
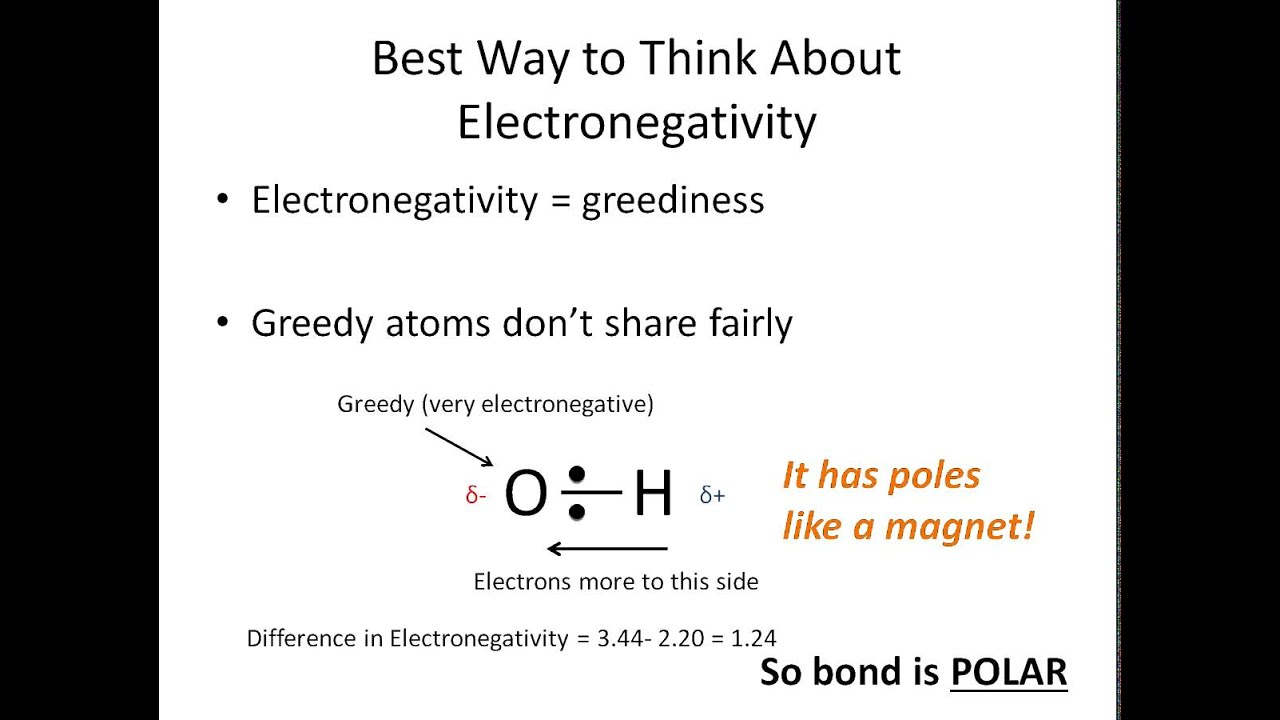
Which Diagram Best Represents A Polar Molecule - Hanenhuusholli

PPT - Chemical Bonding and Molecular Structure (Chapter 9) PowerPoint

How does a polar bond differ from a covalent bond

4 Ways to Determine Bond Polarity - wikiHow

Bond Polarity Chart
Which Bond Is More Polar? - YouTube
Polar and Nonpolar Covalent Bonds - Clear & Simple - YouTube